As endotoxins are exposed within the area of microorganisms, the innate immune process has developed to recognise them to be a danger and also to react accordingly for their existence. Endotoxins are pyrogens, provoking a powerful innate immune reaction. When Gram-adverse germs are killed with the immune procedure, fragments of their membrane containing endotoxins are released during the blood stream and will bring about fever and diarrhoea.
This is particularly legitimate of Reverse Osmosis (RO) techniques. It's been acknowledged that considering that Reverse Osmosis filters are not complete, it may be necessary to have them in sequence so as to manufacture pyrogen-free of charge WFI.
As bacteria evolve and alter to better evade remedy, endotoxins remain a location of continued analyze and investigation.
Nonetheless, endotoxins are also existing in a few concentrations on medical equipment and in intravenous prescription drugs.
Normal processing procedures for Actual physical components of parenteral products including stoppers and vials, deliver for washing these elements with pyrogen-totally free water before sterilization.
Lengthy recognized for a entire world-renowned supplier of substantial purity chemicals and reagents, our corporation proceeds to keep up a happy record of product or service quality and customer service through the institution with the LAL Division, and the introduction of our new PYROSTAR™ ES-File line for the detection of bacterial endotoxin.
This ITG will deal with the importance and interpretation of pyrogen/endotoxin testing. Also resources and methods of depyrogenation might be reviewed. The constraints in the rabbit pyrogen test need to be recognized when examining devices in the course of inspections of sterile drug and system companies.
1 method involves the usage of endotoxin-binding agents, for instance polymyxin B, which can sequester endotoxins and lower their Organic activity. This antibiotic, known for its affinity to lipid A, is frequently utilized in clinical configurations to deal with serious infections a result of Gram-destructive microbes.
Endotoxin testing is only one of numerous types of pyrogen tests necessary inside the food items and Health care House.
Their presence can trigger immune responses, resulting in situations which include sepsis and septic shock, generating them substantial from both equally health-related and microbiological Views.
Gram-detrimental bacteria are characterised by two membranes: the internal membrane surrounds the cytoplasma whereas the outer membrane separates the bacterial cell wall through the external atmosphere.
Endotoxins are everywhere from the surroundings check here and can perhaps trigger problems with chronic publicity. This article will explore the pathogenesis of endotoxins and the way to mitigate their effects over the surroundings.
The Gel-Clot method or gelation measures the quantity of gel formed due to the reaction that happens while in the Amebocyte Lysate within the existence of endotoxins. Inside the hemolymph of your Limulus Polyphemus crab, a series of chain reactions occur as being a reaction to endotoxins, concluding Using the coagulation with the coagulant proteins. This can be a response that can certainly be noticed Using the formation of gel while in the test tube. So as to have the ability to say that the LAL test has offered a constructive end result throughout the Gel Clot method, the tube in which the response has transpired is turned upside down and it is checked click here if the shaped gel keeps independent through the combination immediately after this process.
Neutralizing endotoxins is often a component in controlling bacterial infections and making sure the protection of pharmaceutical products and solutions. A variety of procedures have been created to mitigate the effects of those molecules. These approaches often center on both inactivating endotoxins or blocking their conversation with host cells.
 Destiny’s Child Then & Now!
Destiny’s Child Then & Now! Joshua Jackson Then & Now!
Joshua Jackson Then & Now! Marla Sokoloff Then & Now!
Marla Sokoloff Then & Now! Melissa Joan Hart Then & Now!
Melissa Joan Hart Then & Now!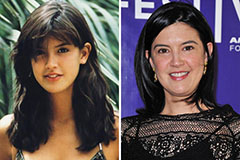 Phoebe Cates Then & Now!
Phoebe Cates Then & Now!